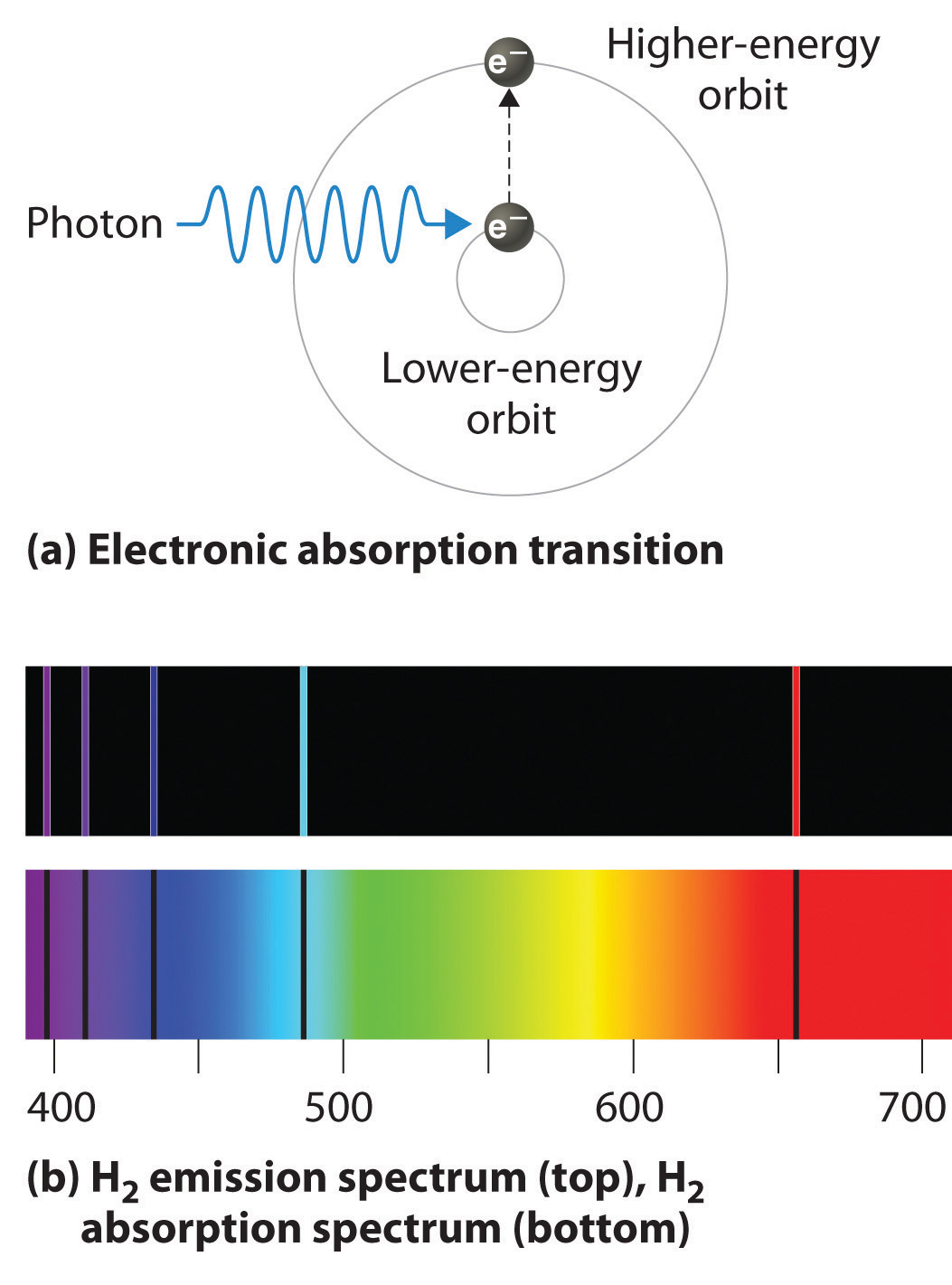
How Are Photons Emitted From An Atom . Web the rydberg equation can be rewritten in terms of the photon energy as follows: Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web photons are emitted by the action of charged particles, although they can be emitted by other methods including radioactive decay. For example, if a red photon of frequency \(f\). We learn how photons are absorbed and emitted by an atom. Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\).
from saylordotorg.github.io
Web photons are emitted by the action of charged particles, although they can be emitted by other methods including radioactive decay. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web the rydberg equation can be rewritten in terms of the photon energy as follows: We learn how photons are absorbed and emitted by an atom. Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. For example, if a red photon of frequency \(f\).
Atomic Spectra and Models of the Atom
How Are Photons Emitted From An Atom Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web the rydberg equation can be rewritten in terms of the photon energy as follows: We learn how photons are absorbed and emitted by an atom. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. For example, if a red photon of frequency \(f\). Web photons are emitted by the action of charged particles, although they can be emitted by other methods including radioactive decay. Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so.
From lovinthings.com
Electrons and Photons Science in Your Life How Are Photons Emitted From An Atom For example, if a red photon of frequency \(f\). Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. We learn how photons are absorbed and emitted by an atom. Web photons are emitted by the action of charged particles, although they can be emitted by other methods including radioactive decay.. How Are Photons Emitted From An Atom.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum How Are Photons Emitted From An Atom Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web the rydberg equation can be rewritten in terms of the photon energy as follows: Ephoton = ryz2(1. How Are Photons Emitted From An Atom.
From www.youtube.com
Bohr Calculation Example 1 find ΔE and the wavelength of the photon How Are Photons Emitted From An Atom Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). We learn how photons are absorbed and emitted by an atom. Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Ephoton = ryz2(1. How Are Photons Emitted From An Atom.
From www.youtube.com
Calculate the wavelength of the photon emitted when the hydrogen atom How Are Photons Emitted From An Atom Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web the rydberg equation can be rewritten in terms of the photon energy as follows: Web. How Are Photons Emitted From An Atom.
From slideplayer.com
Electrons in Atoms, Light and the Periodic Table ppt download How Are Photons Emitted From An Atom Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web the rydberg equation can be rewritten in terms of the photon energy as follows: We learn how photons are absorbed and emitted by an atom. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the. How Are Photons Emitted From An Atom.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts How Are Photons Emitted From An Atom Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web the rydberg equation can be rewritten in terms of the photon energy as follows: Web. How Are Photons Emitted From An Atom.
From www.youtube.com
Find the ratio of energies of photons produced due to transition of How Are Photons Emitted From An Atom Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. We learn how photons are absorbed and emitted by an atom. Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. For example, if a red. How Are Photons Emitted From An Atom.
From www.scienceabc.com
Where Do The Photons Produced By A Source Of Light Come From? » ScienceABC How Are Photons Emitted From An Atom Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). For example, if a red photon of frequency \(f\). Web the rydberg equation can be rewritten in terms of the photon energy as follows: Web photons are emitted by the action of charged particles, although they can. How Are Photons Emitted From An Atom.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps How Are Photons Emitted From An Atom Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. We learn how photons are absorbed and emitted by an atom. Web in the photoelectric absorption. How Are Photons Emitted From An Atom.
From www.slideshare.net
Interaction of photons with matter How Are Photons Emitted From An Atom We learn how photons are absorbed and emitted by an atom. Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web the rydberg equation can. How Are Photons Emitted From An Atom.
From www.doubtnut.com
what is the wavelength of light emitted when the electron in a hydrogen How Are Photons Emitted From An Atom For example, if a red photon of frequency \(f\). Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web photons are emitted by the action of charged particles, although they can be emitted by other methods including radioactive decay. We learn how photons are absorbed and emitted by an atom.. How Are Photons Emitted From An Atom.
From www.youtube.com
ABC Zoom Electrons and photons absorption and transmission of light How Are Photons Emitted From An Atom Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Web the rydberg equation can be rewritten in terms of the photon energy as follows: We learn how. How Are Photons Emitted From An Atom.
From vit-ruengwit.blogspot.com
Ruengwit เเสง How Are Photons Emitted From An Atom Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Web the rydberg equation can be rewritten in terms of the photon energy as. How Are Photons Emitted From An Atom.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 How Are Photons Emitted From An Atom Web photons are emitted by the action of charged particles, although they can be emitted by other methods including radioactive decay. Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Web the rydberg equation can be rewritten in terms of the photon energy as follows: We. How Are Photons Emitted From An Atom.
From pressbooks.online.ucf.edu
29.4 Photon Momentum College Physics How Are Photons Emitted From An Atom Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. We learn how photons are absorbed and emitted by an atom. Web photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Ephoton = ryz2(1 n2 1 − 1. How Are Photons Emitted From An Atom.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps How Are Photons Emitted From An Atom Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in. How Are Photons Emitted From An Atom.
From www.shanghai-optics.com
Lesson 14 How do Atoms Emit Light? How Are Photons Emitted From An Atom We learn how photons are absorbed and emitted by an atom. Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Ephoton = ryz2(1 n2 1 − 1 n2 2) where n1 and n2 are positive integers, n2> n1, and ry is. Web in the photoelectric absorption. How Are Photons Emitted From An Atom.
From schoolbag.info
Rutherford, Planck, and Bohr Atomic Structure Review Training How Are Photons Emitted From An Atom We learn how photons are absorbed and emitted by an atom. For example, if a red photon of frequency \(f\). Web colloquially, one may say that the new, \((n+1)^{\text {st }}\) photon emitted from the atom is automatically in phase with the \(n\). Web the rydberg equation can be rewritten in terms of the photon energy as follows: Web photons. How Are Photons Emitted From An Atom.